Original article: Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis
Published: 13.9.2017, SAGE - Journal of Feline Medicine and Surgery
Niels C Pedersen,1 Yunjeong Kim,2 Hongwei Liu,1 Anushka C Galasiti Kankanamalage,3 Chrissy Eckstrand,4 William C Groutas,3 Michael Bannasch,1 Juliana M Meadows,5 and Kyeong-Ok Chang2
Abstract
The goal
The safety and efficacy of the 3C-Like protease inhibitor GC376 was tested in a group of client-owned cats with various forms of infectious peritonitis (FIP).
Methods
Twenty-four cats aged 3.3 to 82 months (mean 10.4 months) with various forms of FIP were enrolled in the clinical trial. Fourteen cats had wet or mixed FIP and six cats had dry FIP. GC376 was administered subcutaneously every 12 hours at a dose of 15 mg / kg. Cats with neurological symptoms were excluded from the study.
The results
Nineteen of the 20 GC376-treated cats recovered within 2 weeks of starting treatment. However, symptoms of the disease returned after 1-7 weeks of primary treatment, and relapses and new cases were finally treated for at least 12 weeks. Relapses that stopped responding to treatment occurred in 13 of these 19 cats within 1-7 weeks of initial or repeated treatment. Severe neurological disease occurred in 8/13 cats that failed treatment, and abdominal lesions recurred in five cats. At the time of writing, seven cats were in remission. Five kittens aged 3.3–4.4 months with wet FIP were treated for 12 weeks and were in remission for 5–14 months (mean 11.2 months) after treatment and at the time of writing. The sixth kitten was in remission for 10 weeks after 12 weeks of treatment, but relapsed and responded well to the second round of GC376 treatment. The seventh was a 6.8-year-old cat with mesenteric lymph node involvement, who managed to achieve remission after three relapses, which required successively longer repeated treatments for 10 months. Treatment side effects included injection burning and occasional foci of subcutaneous fibrosis and hair loss. In cats treated before 16.-18. week, there was a slow development and abnormal growth of permanent teeth.
Conclusions and significance
GC376 has been shown to be promising in the treatment of cats with specific forms of FIP, opening the door to targeted antiviral therapy.
Introduction
Drugs that directly inhibit viral replication have become key supports in the treatment of chronic viral infections such as HIV / AIDS, hepatitis C virus (HCV), hepatitis B virus, herpesvirus and acute infections such as influenza. RNA viruses, such as HIV-1 and HCV, contain ideal targets for inhibiting viruses, such as RNA-dependent RNA polymerase and protease. Proteases are a particularly good target because they are involved in virus maturation (HIV) or in the production of functional viral proteins (HCV). Protease inhibitors are also used in combination with reverse transcription inhibitors in the lifelong treatment of HIV / AIDS. Combinations of different protease inhibitors are highly effective in treating HCV infection in humans. Therefore, it is not surprising that viral protease should also be an attractive target for research into animal infections caused by RNA virus. Kim et al. synthesized peptidyl compounds that target 3C-like (3CLpro) proteases and evaluated their efficacy against feline coronavirus (FCoV) and feline calicivirus, as well as important human RNA viruses that encode 3CLpro or a related 3C protease. They identified a series of compounds that showed strong inhibitory activity against various coronaviruses, including FCoV, with a high safety margin. The efficacy of their 3CLpro inhibitors has been tested in mice infected with hepatitis A59 virus, murine coronavirus, and has been found to cause significant reductions in virus titers and pathological lesions.
There are currently no commercially available antiviral drugs for coronavirus infections in humans or animals, and studies by Kim et al. demonstrated that inhibition of 3CLpro could lead to suppression of coronavirus replication in vivo. They have shown that some of their 3CLpro inhibitors are useful as therapeutic agents against these important viruses in domestic and wild cats. This was confirmed by a study using experimental infection with feline infectious peritonitis virus in laboratory cats. Although experimental FIPV infection is highly fatal, once the infection has reached a definable stage, 14-20 days of GC376 treatment resulted in rapid remission of the disease in six cats, which lasted more than 12 months at the time of publication.
Materials and methods
Official protocols
This study was conducted in accordance with Protocol 18731 approved by the Institutional Committee on the Care and Use of Animals and the Clinical Trials Evaluation Committee of the Veterinary Medical Teaching Hospital at the University of California, Davis. This protocol details the testing conditions for the new protease inhibitor GC376 in client-owned cats. Each owner was required to read and agree to the study conditions.
Organization of a clinical trial
The subject of the study was the evaluation of the 3CLpro GC376 inhibitor in a group of cats with naturally occurring FIP. The study did not include the placebo group because, as Miller and Brody noted, "the main ethical principle in placebo-controlled clinical trials is that if there is a proven effective treatment for a given condition, testing against placebo is unethical." GC376 has already been shown to be highly effective in the treatment of cats with experimentally induced FIP prior to this study, suggesting an existing effective treatment. The placebo control was replaced by a group with a naturally occurring disease. None of the 20 treated cats showed persistent beneficial responses to the treatment they received prior to GC376 treatment.
The institutional rules precluded the use of cats obtained from shelters or similar research facilities of this type, and required that all cats be legally owned / adopted and treated with the express consent of the owner. Cats with clinically obvious neurological disease were excluded. The study eventually included 20 cats from different areas of the United States, of different ages, and with various forms of FIP. This relatively small group of cats provided valuable insights into the design of the trial, interaction and compliance with the owner, safety and efficacy monitoring, determination of the minimum dosing regimen, evaluation of disease relapses during or after treatment, and determination of clinical forms of FIP that are most appropriate for treatment. This information will hopefully assist in further testing needed for the licensing and possible commercialization of GC376 and for performing similar tests on future antiviral drugs for FIPV and other chronic feline viral infections.
Test group description
Twenty cats and their owners were included in the experiment, and the relevant information for each cat is shown in Table 1 and for the entire experimental group in Figure 1. The cats were enrolled in the study with varying degrees of preliminary testing by primary care veterinarians. This testing usually involved a complete blood count (CBC) with total plasma protein, globulin (G), albumin (A), A: G; serum chemical profile and effusion analysis, including total protein, actual or estimated cell numbers and inflammatory cell type. A small proportion of the cats underwent additional testing, which included FIPV antibody titers, abdominal or thoracic ultrasound, affected tissue biopsies, and real-time quantitative PCR (qRT-PCR) from the effusions.
Table 1
Basic data, origin, main clinical signs and main findings at autopsy after treatment with protease inhibitor GC376
| ID / Name | Age (months) | Weight (kg) | Gender | Tribe | Origin | Symptoms | Condition | Autopsy |
|---|---|---|---|---|---|---|---|---|
| CT01 (Echo) | 5.6 | 1.64 | FS | DSH | KR | Peritonitis, stunted | - | B, Int |
| CT02 (Cate) | 6 | 2.67 | FS | DLH | KR | Peritonitis, stunted | - | B, E, Int, L, MLN |
| CT03 (Pancake) | 7.86 | 3.18 | MC | Him | CT | Dry (Col) to moist | + | Int, L, MLN, S, Om, P |
| CT04 (Kratos) | 82 | 4.8 | MC | DSH | KR | Dry (MLN) | Remission | |
| CT05 (Scooter) | 10 | 4.25 | MC | DSH | KR | Dry (E, MLN, K) | - | B, E, L, K, MLN |
| CT07 (Mac) | 6.6 | 2.6 | MC | DSH | KR | Dry (Col) | + | E, Int, L, MLN, S, K, A, Lu |
| CT08 (Phoebe) | 4.2 | 2.18 | FS | DSH | KR | Dry (E) | - | B, E, K, MLN, S |
| CT09 (Sammy) | 10.5 | 2.89 | MC | DSH | KR | Dry (MLN, K) | ?* | B* |
| CT10 (Bandit) | 17.9 | 4.06 | MC | Him | CT | Dry (Col) to moist | + | B, E, Int, L, MLN, K, Om, P, Lu |
| CT12 (Daisy) | 7.5 | 2.5 | FS | DSH | KR | Peritonitis, stunted | - | B, Int, L, S |
| CT13 (Leo) | 7.4 | 1.97 | MC | Sphynx | CT | Dry (E, K) | + | B, E, Int, L, MLN, S, K |
| CT14 (Muffin) | 8 | 2.94 | FS | DSH | KR | Dry (Col) to moist | + | E, Int, L, MLN, K, Om, P |
| CT15 (Flora) | 4.3 | 2.39 | F | DSH | FC | Peritonitis | Remission | |
| CT16 (Bean) | 4 | 1.4 | FS | DSH | KR | Peritonitis, stunted | + | B,† E, Int, L, MLN, S, Om, P |
| CT17 (Peanut) | 4.4 | 2.3 | M | DSH | KR | Peritonitis | Remission | |
| CT18 (Smokey) | 4 | 1.84 | MC | DSH | KR | Peritonitis | Remission | |
| CT20 (Cloud) | 3.3 | 1.55 | M | RM | CT | Pleuritis (MLN) | Remission | |
| CT21 (Phoebe) | 4.8 | 1.92 | F | DSH | KR | Peritonitis | Remission / relapse / re-treatment | |
| CT22 (Pepper) | 3.3 | 1.6 | F | Siberian | CT | Peritonitis | + | B, E, Om, MLN, Lu, Dia |
| CT23 (Oakely) | 3.9 | 3.1 | FS | DSH | KR | Peritonitis | Remission | |
| Average | 10.28 | 2.59 | ||||||
| SD | 17.22 | 0.94 |
* No autopsy was performed, but terminal neurological symptoms were present
† Severe cerebral edema, no typical inflammatory lesions have been reported

Demographics of study cats. (a-c) Pie charts summarizing the percentage of patients: (a) age in months, (b) breed, or (c) origin. (d) Bar graph showing forms of feline infectious peritonitis (FIP) enrolled patients.
M = months; DSH = domestic shorthair; DLH = domestic longhair; MD = Maryland; OH = Ohio; Tx = Texas; FL = Florida; IL = Illinois; CT = Connecticut; CA = California
Cats with clinical signs of neurological impairment were excluded from the study based on previous unpublished experimental studies with GC376. One cat that survived a previous study of the pharmacokinetics and efficacy of GC376 had a recurrence of FIP with neurological symptoms 6 months after appearing to be a successful treatment for acute infection.6 This cat did not respond to a repeated GC376 penetration study that prompted a penetration study. to the brain. GC376 levels in laboratory cat brain represented only 3% plasma drug concentrations.
Confirmation of the disease
The diagnosis of FIP was confirmed at study entry based on baseline data, clinical history, examination of previous laboratory tests, physical examination, and repetition of baseline blood and effusion tests. Manual abdominal palpation was usually sufficient to identify ascites, enlarged mesenteric lymph nodes, appendix enlargement and associated ileocecal-colic lymph nodes, renal tumors, and colonic infiltration. Manual palpation was supplemented with ultrasound if necessary. The eyes were initially examined with direct light for any abnormalities in the retina, for clots in the anterior chamber or on the back of the cornea, and flashes in the ventricular water. The presence of ocular disease was confirmed by a complete ophthalmoscopic examination performed by the Ophthalmological Service of the Veterinary Medical University Hospital (VMTH), UC Davis. The presence of FIPV was further confirmed by qRT-PCR, either from abdominal or thoracic effusions collected at the time of admission or at the time of necropsy. Sequencing of the FIPV protease gene was performed on cats that relapsed during treatment to determine if there was a potential mutation causing drug resistance.
The diagnosis of dry-wet (mixed) FIP in three cats (CT03, CT10 and CT14) was based on diffuse colon enlargement and a history of loose stools, blood and mucus in the stools, defecation strains and small caliber stools before abdominal effusions. Colon FIP has been described as a specific variant form of non-fusive FIP. Mixed FIP was also suspected in cats CT01, CT02 and CT12 due to growth arrest, which preceded the occurrence of abdominal effusions by many weeks.
Treatment regimen
GC376 was synthesized in highly pure form and prepared at a concentration of 53 mg / ml in 10% ethanol and 90% polyethylene glycol 400 as described above. GC376 was administered subcutaneously (SC) at a dose of 15 mg / kg every 12 hours of SC, unless otherwise stated. The effective dose for cats with experimentally induced FIP was 10 mg / kg / q12 h SC, but the dose was increased to 15 mg / kg after the first cat (CT01) did not respond to the lower 10 mg / kg dose determined by previous pharmacokinetic studies. It was a clinical decision based on this cat's response to treatment.
Monitoring response to treatment
Based on preliminary testing and initial evaluation at the time of presentation at UC Davis, cats with FIP were hospitalized for at least 5 days and started treatment immediately. They were examined in detail for rectal temperature, pulse, respiration, appetite and activity at least twice a day. A clumping litter was used to allow daily evaluation of stool volume and consistency and urination. Whole blood was collected into EDTA or heparin by venipuncture before treatment, at two-day intervals during hospitalization, at discharge time, and at two-week intervals during the first month and at monthly or longer intervals thereafter. Routine blood tests at each time point included minimal hematocrit, total plasma protein, icteric index, total white blood cell count, differential white blood cell count, and absolute neutrophil, lymphocyte, and monocyte and eosinophil counts. To check the potential toxicity of the medicines, blood serum chemistry values were recorded regularly. Abdominal effusion samples were obtained by paracentesis every other day if they could be obtained, which was usually the first 3-7 days. Cats with shortness of breath were examined by thoracic ultrasonography and a fluid sample was obtained by ultrasound-guided paracentesis. The effluents were examined for the presence of fibrin clots, neutrophil and small / large mononuclear cell admixtures, yellowing intensity, fiber viscosity, and total protein content. Cell pellets from peritoneal or thoracic effusions were also examined by qRT-PCR for viral RNA levels as previously described.
Cats were issued to their owners when a positive response to treatment was noted, usually within 5 days. The owner (s) were instructed by either the chief veterinarian or the primary care veterinarian how to administer the drug twice daily by subcutaneous injection. The injection sites were varied to include the upper line from the nape of the neck to the middle of the back and to the sides of the chest and hips. Care was taken to avoid storing the drug in the dermis or gradually in the same subcutaneous site. Owners were encouraged to maintain daily records of rectal temperature, activity, appetite, defecation and urination, and weekly to biweekly body weights. Periodic blood samples for CBC and serum chemistry were collected by the owners' personal veterinarians and sent to commercial veterinary diagnostic laboratories. Any abnormal symptoms or behavior should be noted and reported immediately. Euthanasia was performed at either UC Davis or a primary care veterinarian, as appropriate. The bodies of the cats killed by the primary care veterinarians were immediately cooled and sent in ice packs by express mail to UC Davis for autopsy. The owners' requirements for treatment and final disposition of the body were respected.
The results
Determination of treatment duration
The first five cats in the study were initially treated for 2 weeks (CT01, CT02, CT03, CT04 and CT05). A rapid improvement in health was observed in all cats and treatment was discontinued. Despite a favorable initial response, symptoms of the disease were repeated 1 (CT01, CT05), 2 (CT03, CT04) or 7 (CT02) weeks after the end of the 2-week treatment (Figure 2). The cats were then treated again, due to a gradual prolongation of the primary and secondary treatment time until their FIP remained sensitive to GC376 (see CT04, CT22, Figure 2). New cats that entered the study were further treated for 3 (CT07) or 4 weeks (CT08, CT16). Cats CT08 and CT16 initially responded, but their symptoms reappeared during treatment. Cat CT08 developed neurological disease, while cat CT16 had recurrent abdominal lesions (Table 1). Primary and secondary treatment times were then extended to 9 weeks (CT07, CT09, CT10, CT14) (Figure 2). Cat CT09 developed neurological symptoms during the 9-week basic treatment and was eventually sacrificed when the symptoms of the disease became severe. CT07 developed neurological disease 6 weeks after the start of the second treatment. From this point, all new cats were accepted into the study and older cats, such as CT10, were treated or re-treated for at least 12 weeks. The benefit of 12 weeks of treatment was most evident in the CT04 cat, which had previously been treated three times with a shorter treatment period followed by relapse (Figure 2). Treatment was discontinued in cats that had no clinical or laboratory signs of disease after 12 weeks of primary or secondary treatment. It was found that the minimum treatment period should be around 12 weeks. Cat CT21 was treated for 17 weeks due to delayed improvement in total protein and white blood cell counts (Figure 2). This cat relapsed pleural FIP 13 weeks later and underwent further treatment at the time of writing.

Treatment time scale and clinical outcome of 20 cats that entered the clinical study with the protease inhibitor GC376. The periods during which the cats were treated are indicated by solid lines. The date of the last day of treatment is given for six cats that have achieved permanent clinical remission. Cat 21 was still in treatment at the time of writing. The remaining 13 cats succumbed to non-neurological FIP or neurological FIP after completion of primary or secondary treatment within 0-7 weeks.
Response to initial treatment and indicators of favorable response
During the first 1-4 weeks of treatment, 19/20 cats showed a dramatic and progressive improvement in health. The exception was the CT16 cat, which responded by a drop in rectal temperature during the first 4 days of treatment. However, the fever returned and the health continued to deteriorate over the next 23 days and the cat was euthanized. The fever (> 38.9 ° C) in the other 19 cats disappeared within 24-48 hours, with an improvement in appetite, activity, growth and weight gain. Abdominal effusions were usually undetectable within 2 weeks. The residual thoracic effusion remaining after the initial therapeutic drainage after 3 days in the CT20 cat almost disappeared. Renal tumors in cats CT02 and CT13 also shrunk rapidly and were no longer palpable after 2 weeks. Enlarged mesenteric lymph nodes returned to normal size more slowly. The palpable colon thickening and associated ileo-cecal-colic formations responded the slowest and persisted in the CT03 cat despite treatment and a return to an otherwise normal state of health. Jaundice, a common finding in younger cats with effusive FIP, slowly resolved over 2 weeks or more, with a reduction in hyperbilirubinemia. Symptoms of ocular disease began to resolve within 48 hours and resolved within 1 week, regardless of initial severity (Figure 3).

Appearance of CT08 cat eyes before treatment (a) and one week later (b). This cat developed severe neurological symptoms 3 weeks after the start of treatment.
Weight gain was a simple and accurate criterion for growth and health improvement. The weight of cat CT04, the oldest cat in the test, was used as a reference value for this parameter (Figure 4a). Cat CT04 showed a significant weight loss of 30%. She gained weight after each round of treatment, began to lose weight shortly before each relapse, and gained weight again after each treatment. After 9.3 months without treatment and after treatment (4.8 kg to 7.19 kg), she regained all her lost weight. All kittens with permanent remission during and after antiviral treatment gained weight continuously, indicating that normal growth continued with antiviral treatment (Figure 4b). One cat (CT15) and two cats (CT17, 20) were castrated without complications during disease remission.

Antiviral treatment and weight changes. (a) Cat CT04, a 6.8-year-old castrated male who had dry feline infectious peritonitis (FIP), underwent four cycles of antiviral treatment with increasing duration, as shown by the dotted boxes. He lost weight before each relapse and gained weight after subsequent treatment. (b) Weight gains in four kittens aged 3.5-4.4 months during and after antiviral treatment are indicated by dots. The dotted rectangle indicates the length of antiviral treatment (12 weeks)
Lymphopenia was a common clinical symptom in cats with wet FIP (Figure 5) and tended to directly correlate with the severity of abdominal inflammation, as indicated by viscosity, presence of fibrin fibers, protein content, cell number, and yellowness of the effusion. Lymphopenia improved in the treatment of all cats with wet FIP except CT16, but did not help predict disease relapses that occurred afterwards (Figure 5a). In cats with dry FIP, lymphopenia was not as severe and was not as useful as other parameters in assessing response to treatment (Figure 5b).

Mean and standard deviation (SD) of absolute lymphocyte counts in treated patients with (a) wet or (b) dry feline infectious peritonitis (FIP). (a) Twelve cats (empty ring) showing abdominal or thoracic effusion and treated for up to 12 weeks. The thirteenth cat (CT16, full ring) with abdominal effusion responded poorly to treatment. b) Seven cats with dry or wet form of FIP and subsequent treatment for 6 weeks.
Total plasma protein levels as an indirect indicator of globulin concentration were often increased on examination, but values were highly variable during the first 4 weeks and often increased transiently during effusion resorption. Cats that ultimately failed treatment tended to have higher total plasma protein concentrations at the start of treatment and tended to maintain higher levels during treatment than cats that successfully achieved permanent remission (Figure 6).
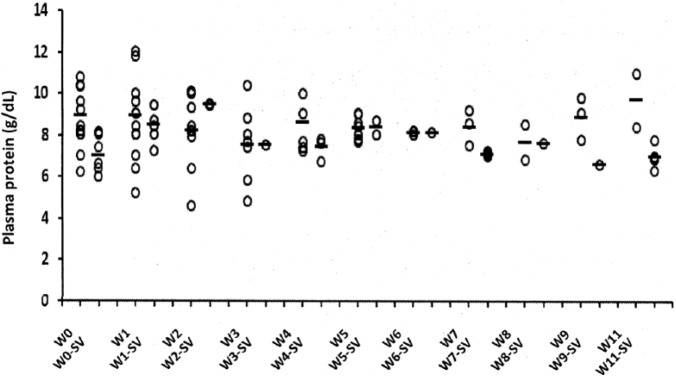
Mean and SD of total plasma protein levels in 20 cats over a 12 week period. Thirteen cats suffered fatal relapses at different weeks of treatment (W) and seven cats achieved permanent remission after 12 weeks of treatment (W-SV)
Decreased viral RNA levels in ascitic fluid cells in association with treatment
Sequential ascites samples were taken from several identical cats during the first 6-25 days of antiviral treatment and tested for viral RNA levels by qRT-PCR. FIPV levels are often low or negative in the blood of cats with FIP and are highest in effusion cells. Therefore, cells from ascites or pleural effusions were the most reliable source of FIPV RNA. Cats CT15, CT16 and CT17 had 955, 1699 and 2937-fold higher levels of viral RNA, respectively, than CT02, which had the lowest viral load in pre-treatment effusion (Figure 7). Viral RNA levels decreased up to 1567463-fold over 2 weeks compared to pre-treatment values, except for the CT16 cat (Figure 8), which had the second highest pre-treatment viral RNA level among the 12 cats with effusion samples available for testing (Figure 7). The absence of a rapid decrease in viral RNA levels in the CT16 cat, together with severe lymphopenia, may explain why it did not respond to treatment. CT10 also had a slightly slower decrease in virus levels and relapsed twice after antiviral treatment. Notably, viral RNA levels in ascites cells in CT15, CT17, and CT18 cats declined most rapidly and were also among the five cats that experienced permanent remission of the disease. Whether the cause was a property of individual FIPV isolates or the form and severity of the host disease was not determined.

Relative initial levels of feline infectious peritonitis virus (FIPV) RNA in patient effusions prior to antiviral therapy. Real-time quantitative PCR was performed on pre-treatment effusion patient samples. Relative baseline viral RNA levels as fold differences compared to viral levels before treatment with CT02, the cat with the lowest RNA levels. RNA transcript levels were calculated for each patient using the ∆Ct method with the beta-actin reference gene

Reduction of feline infectious peritonitis virus RNA from sequential effusion samples during GC376 treatment in cats CT10, CT12, CT15, CT16, CT17, CT18 and CT23. Each point indicates a fold reduction in viral RNA levels compared to pre-treatment levels (day 0). Viral RNA levels were determined by real-time quantitative PCR using the ∆Ct method and beta-actin reference gene
Treatment failure due to recurrence of abdominal FIP or the occurrence of neurological disease
Thirteen of the 20 cats in the test eventually relapsed. One cat (CT16) did not show significant improvement and was euthanized 3 weeks after the start of the 4-week treatment regimen (Figures 2 and 8), 8), while the other 12 had a variable period of disease remission after primary or secondary treatment lasting 3-17. weeks (mean 7.8 weeks) (Figure 2). All but one of these 13 cats (CT09) were necropsied (Table 1). Eight of these cats were euthanized for severe neurological symptoms and five for recurrent abdominal disease (Figure 2). Three cats that underwent neurological disease (CT05, CT08, CT13) due to ocular FIP were secondary to the examination (Table 1). The earliest symptoms of neurological disease included fever, which persisted despite continued treatment, apathy, occasional muscle twitching of the ears and muscles, unusual swallowing movements, compulsive limb twitching, and loss of normal mentality, manifested by brief episodes of numbness or numbness. These symptoms persisted during treatment for several days or weeks, but eventually led to incoordination and tonic / clonic seizures. The incidence and rapid progression of neurological symptoms after discontinuation of treatment were more pronounced than during treatment (Figure 2).
Five cats (CT03, CT07, CT10, CT14 and CT16) had a recurrence of typical intra-abdominal lesions in the absence of neurological symptoms during or after treatment (Table 1). Four of them had ileocecal formations (CT03, CT07 and CT14) or an enlarged colonic lymph node (CT10) that had shrunk (CT03, CT10 and CT14) or were no longer palpable (CT07) after primary treatment. However, CT03 continued to suffer from severe constipation, strain, and toothpaste-like stools. The severity of colonic obstruction required resection of the colon, which alleviated clinical symptoms but did not prevent a recurrence of abdominal disease. All three cats that developed severe ileocecal infiltrates still showed evidence of this form of FIP at necropsy, and immunohistochemistry showed FIPV antigen in macrophages in granulomatous inflammation (Figure 9).

An incision from the severely thickened wall of the resected colon from cat CT03. Immunoperoxidase (brown) stained for feline antigen of infectious peritonitis virus is observed in macrophages around the periphery of the granulomatous lesion. Persistence of the virus in the colon occurred during treatment and remission of other symptoms of the disease (eg effusive peritonitis)
Attempts to treat a neurological disease by increasing the dose of the drug and prolonging the duration of treatment
To alleviate neurological symptoms, we tried to increase the dose of GC376, thereby increasing its blood level and the amount of drug that crossed the blood-brain barrier. Cat CT01 showed effusion FIP and was initially treated with GC376 (10 mg / kg q12h SC for 9 days). The cat responded well, but on day 9 the fever returned and the dose was increased to 15 mg / kg every 12 hours for 5 days. The fever disappeared and treatment was stopped on day 14. Three days later, the fever returned along with indeterminate neurological symptoms consisting of muscle twitching, abnormal limb stretching, and abnormal swallowing movements. The cat was immediately re-administered a dose of 15 mg / kg every 12 hours of SC and its condition improved, but soon after it worsened with the return of fever and the same vague neurological symptoms with mild incoordination. The dose was then increased to 50 mg / kg every 12 hours of SC for 14 days and improved to near normal. Treatment was stopped, but neurological symptoms returned immediately. The cat was then treated for another four days with a dose of 50 mg / kg q12 h SC, during which the neurological symptoms improved again. However, the decision was made to stop the treatment completely. The cat's condition remained stable for 1 week and then developed extreme incoordination, dementia and tonic / clonic seizures. Euthanasia was performed and autopsy showed lesions only in the brain.
Cat CT12 responded well to treatment at 15 mg / kg q12 h SC; rectal temperature returned to normal within 48 hours and abdominal effusion disappeared within 2 weeks. The cat appeared normal after the second week of treatment, but then had a persistent fever of 38.9-40 ° C. The owners felt that the cat otherwise had normal activity and appetite, so the treatment continued at the same dosage. However, the fever persisted, mild signs of behavior change were observed, and the cat did not grow as expected. The cat continued treatment for another 15 weeks, during which the drug dose was temporarily reduced twice (ie to 10 mg / kg every 12 hours and 15 mg / kg every 24 hours) for several days, but the fever increased and the activity decreased each time. Dosing of 15 mg / kg was resumed every 12 hours. The cat continued to show signs of variable fever and unclear signs of behavior, but the owners were optimistic about the cat's appetite and activity level. The treatment of the cat was then stopped because further use of the drug for this purpose could not be justified. The cat's condition remained unchanged with persistent fever, solitary behavior and stunted growth for another 5 weeks. At week 22, severe neurological symptoms consisting of incoordination, dementia and seizures appeared and the cat was euthanized. Macroscopic and microscopic lesions were limited to the brain.
Viral resistance testing
The development of a drug-resistant virus was considered in the CT03 cat, which relapsed with abdominal lesions after an initial favorable response to the treatment of granulomatous colitis and mixed FIP. At the time of necropsy, granulomatous lesions were still present in the abdominal cavity and no macroscopic or microscopic lesions were found in the brain (Table 1). Therefore, disease recurrence was not associated with neurological disease and FIPV persistent antigen was identified in macrophages in granulomatous lesions. Sequence comparisons were made between 3CLpro from pre-treatment effusion and from the omentum taken at autopsy 95 days later. However, no amino acid substitutions were found in 3CLpro, suggesting that the presence of drug-resistant virus was not the cause of recurrent cat disease.
Pre-treatment 3CLpro viral RNA sequences were also compared with those obtained 25 days (CT16), 139 days (CT02), 149 days (CT12) and 231 days (CT10) later at necropsy. No differences in 3CLpro were observed during this period. The sequences also remained unchanged for CT02, CT16 and CT12 from the time of admission to necropsy. CT10 lung and spleen viral 3CLpro, which relapsed twice for 8 months and was re-treated, showed an Asp-to-Ser substitution at position 25 and a Lys-to-Asp substitution at position 260 compared to the pre-treatment abdominal fluid virus. . The exact effects of these mutations on protease function are currently being investigated. Genetic evolution of quasi-viral proteins has been reported to occur over time in patients chronically infected with RNA virus (HCV) and may lead to sporadic amino acid changes.
Occurrence of permanent clinical remissions
Seven of the 20 cats in the GC376 treatment study, all of whom underwent at least 12 weeks of continuous treatment, were categorized as potential treatment successes based on more than 12 weeks of disease remission after cessation of treatment (Figure 2). Six of these kittens had an acute abdominal effusion (CT15, CT17, CT18, CT21, CT23) or pleural (CT20) disease at 3.3-4.4 months of age and were treated continuously for 12 or 17 (CT21 cat) weeks (Table 1, figure 2). The seventh cat (CT04), a 6.8-year-old cross-castrated male with dry FIP restricted to the mesenteric lymph node, also achieved long-term remission, but only after four treatment cycles of increasing duration (Table 1, Figure 2).
Six of these long-lived cats had abnormalities in CBC, hematocrit, and total proteins at the start of treatment, but had completely normal blood levels at the time of treatment. However, the CT21 cat still had elevated plasma protein levels and an increased white blood cell count after 12 weeks and continued treatment for another 5 weeks. Plasma protein and white blood cell counts improved after another 5 weeks of treatment, but were still not within the reference range. Thirteen weeks after the end of treatment, the cat developed a typical FIP chest effusion with fever. Chest fluid was aspirated to improve respiration, and the cat began a second round of GC376 and at the time of writing was afebrile, active, and ate after 8 weeks of treatment. The treatment will last for 12 weeks if there are no signs of the disease again.
Side effects observed during and after treatment
Two side effects were observed during and after GC376 treatment. The drug often caused stinging / burning when injected. Subcutaneous edema occurred when too many injections were given at the same site, but they resolved rapidly. One cat (CT12) experienced deeply localized ulceration between the scapulae at approximately week 14 of the 18-week treatment period. However, no evidence of dermal FIP was observed at necropsy and was likely a response to continuous injections at the same site. A survey of seven long-lived cats showed appreciable focal subcutaneous thickening. In one cat, four calcified peas the size of peas appeared on X-rays. These lumps were surgically removed along with the surrounding fibrotic tissue. The other three long-term survivors have 1-3 small focal areas with permanent hair loss at the injection sites, which are covered by the surrounding fur (Figure 10). The owners and their veterinarians were asked to check these lesions regularly for any changes or the appearance of new lesions.

Focal area of permanent hair loss caused by unwanted deposition of GC376 in the epidermis of cat CT21. These areas were usually covered with hair and were not visible from the outside
The most significant side effect associated with long-term treatment was juvenile teeth. Normal formation, growth and incision of permanent teeth were delayed in all four treated kittens aged 3.3-4.4 months. The ocular teeth, incisors, fourth premolar and molar were the least affected, while the second and third premolar were the most affected (Figure 11). Adult teeth appeared smaller than normlingual, and this, together with the delayed cutting, led either to the retention of the deciduous canines, the failure of the deciduous teeth or the partial incision of the abnormal permanent teeth to the concurrent deciduous teeth. No other anatomical or physiological defects were observed in any of the long-term survivors and no autopsy was observed.
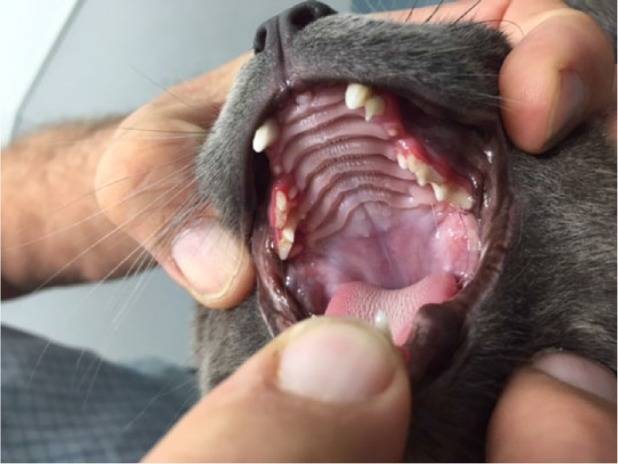
Adult teeth of a CT17 cat who was treated with GC376 for 12 weeks, starting at 4.4 months of age. The upper left milk canine is visible. The upper second and third premolars appear to be milky. The small permanent third premolars were partially cut lingually to the dairy third upper premolars. The gingiva surrounding the left canine and premolars is inflamed. Adult canines also appear smaller than usual. The permanent right canine and fourth upper premolar appear to have cut normally
Autopsy findings
The bodies of 12/13 cats that did not succeed in treatment were necropsied, including a rough and histological examination and immunohistochemistry of the affected tissues for FIPV antigen. Tissues collected and examined included representative sections of all major abdominal and thoracic organs, brain and eyes. The rough examination identified three different presentations. Five cats did not show significant signs of active FIP (CT01, CT02, CT05, CT08, C12), three had lesions corresponding to non-fusion FIP (CT07, CT10, CT13) and four had effusion peritonitis with multiorgan involvement (CT03, CT14, CT16, CT22). The histology of the five cats, which did not show sufficient evidence of the disease, showed mostly mild mononuclear infiltrates, usually perivascular inflammation in the eye, liver, intestinal wall and kidneys. Three cats with non-fusion FIP had mild to severe inflammation in many organs with the most severe lesions in the eye, mesenteric lymph nodes, kidneys, and lungs. Three cats with effusion FIP had severe pyogranulomatous inflammation in several abdominal organs, including the omentum, peritoneum, intestinal wall, mesenteric lymph nodes, liver, and spleen.
Severe inflammation typical of cerebral FIP was present in the brains of all but one (CT07) of the eight cats that underwent necropsy without significant signs of FIP or with non-fusion FIP. One cat without characteristic brain lesions had severe cerebral edema. In contrast, typical FIP lesions were absent in the brains of all three dissected cats with effusive FIP. Stereotypic FIP brain lesions were characterized by moderate to severe chronic meningoencephalitis and ventriculitis associated with periventricular necrosis of the parenchyma (Figure 12a). The fourth ventricle was the most severely affected, and meningitis was most often observed ventrally into the cerebellum and brainstem. Strong perivascular cuffs associated with vasculitis have often been observed. FIP antigen was demonstrated by immunoperoxidase staining in the brain of 6/7 cases of stereotyped cerebral FIP (Figure 12b). Tissues from 11 dissected cats were tested for the presence of FIPV RNA by qRT-PCR. All showed a positive result, thus determining the persistence of the virus in cats in which treatment was not successful.

Photomicrographs of lesions in cat brain CT08. This cat developed a severe neurological disease during the initial treatment of GC376. (a) The fourth chamber contains protein fluid mixed with numerous neutrophils and macrophages that interfere multifocally with the surrounding dilute neuropil. Large cuffs of lymphocytes and plasma cells surround blood vessels (*) (hematoxylin staining, 20x magnification). (b) Several cells resembling peritoneal macrophages (marked small area in Figure 12a) show positive immunoreactivity for feline infectious peritonitis antigen (hematoxylin contrast dye, 600x magnification).
Discussion
Success in the treatment of GC376 in experimental FIPV infection motivated us to examine the efficacy of GC376 in naturally developed FIP. There are significant differences between experimental fusion abdominal FIP and naturally occurring disease. The experimental disease bypasses the critical initial stage, which begins in kittens by exposure to the harmless feline enteric coronavirus (FECV). Naturally occurring FIP is the result of specific mutants that arise after FECV infection, and FIP occurs in the presence of immunity to FECV. In contrast, experimental FIP is induced in cats that have not encountered coronavirus by intraperitoneal injection of a large dose of purified FIPV obtained from a laboratory cat. The naturally occurring disease is often subclinical for many weeks or months before observing external signs of the disease, while experimental symptoms of the disease appear within 2-4 weeks and progress rapidly. Naturally occurring FIP has various clinical forms, while experimental infection almost always has an abdominal effusion form. FIP in nature is also affected by the environment of disease-increasing cofactors, while experimental disease occurs in cats without external influences. Differences may explain why only a small proportion of cats naturally exposed to FIPV develop the disease, while 80-100% experimentally infected cats die. Our predictions proved to be correct, and naturally occurring FIP was much more difficult to treat than experimental disease. However, it should be emphasized that this experiment would not have been approved without the information obtained from pharmacokinetic studies, acute and chronic toxicity and efficacy studies performed in laboratory cats.
It was the first attempt to use a targeted antiviral drug against a systemic and highly fatal veterinary disease. Although no specific antiviral drugs for coronavirus infections in humans or animals are yet available, antiviral drugs for other human viral infections, such as HCV and HIV-1, have been developed for treatment and the use of these drugs has provided a solid basis for their application to animal diseases such as FIP. HCV mainly infects liver cells and causes a persistent viral infection in most people. However, only about 20-30% of them develop liver disease within a time horizon of 20-30 years. HCV infection can be eliminated by non-specific antiviral therapy (interferon and ribavirin) for 6-12 months in approximately half of the patients, and the recent introduction of direct-acting antiviral drugs for 3-6 months significantly increased the cure rate to more than 90% during treatment. HIV infection in humans leads to a prolonged asymptomatic condition and eventually to advanced HIV disease. HIV-1 infects T cells and macrophages and survives in a latent state. More than 30 antiretroviral drugs, most of which are used in combination with two or more drugs, have been used successfully to reduce the viral load to undetectable levels in the blood of HIV / AIDS patients. However, the virus returns after discontinuation of antiviral treatment and thus requires lifelong antiviral treatment. The spread of the virus to the brain, which is mainly mediated by virus-infected macrophages, and the subsequent development of neurological disease occur in more than 50% HIV infections. Therefore, neurological deterioration still remains an important problem at this time of antiviral treatment. These precedents for antiviral treatment of HCV and HIV-1 infections indicate that treatment outcome (viral clearance vs. viral persistence), duration of treatment (final vs. continuous) and the presence of neurological sequelae are strongly influenced by viral pathogenesis.
This study was limited to 20 cats with FIP, which represented the spectrum of age and forms of the disease. Although the number of cats treated was small, a surprising amount of information was gathered, such as how long to be treated, possible side effects, how to identify the clinical form of FIP that is most likely to respond to treatment, and potential indicators. treatment failure and its success. The clinical study was based on experience gained from pharmacokinetic and efficacy studies performed in laboratory cats. Based on experimental studies, the initial treatment period was set at 2 weeks, but was eventually extended to 12 weeks or more based on experience gained during testing. This final treatment period was close to 3-6 months used to treat HCV infection in people with direct-acting antiviral drugs. Based on experimental studies, difficulties in treating neurological forms of the disease have been expected. Side effects were acceptable and included injection burning and dermal and subcutaneous inflammation when too much drug was administered to the same sites. This phenomenon has previously been observed in laboratory cats. A more serious side effect not previously observed in laboratory cats was limited to kittens and included slowed development of adult teeth and retention or delayed loss of deciduous teeth.
GC376 treatment was successful in inducing significant remission of disease symptoms and regression of lesions in 19/20 cats. This result confirms our findings of a rapid reversal of clinical signs in laboratory cats with experimental FIP treated with GC376, and expanded our knowledge of the effects of the drug on a wide range of forms of naturally occurring FIP. The cats came from different parts of the United States and even Peru, confirming that the geographically diverse strains of FIPV were equally sensitive to this inhibitor. Significant reductions in viral RNA transcripts in effusions occurred within a few days of treatment, along with a rapid improvement in health. However, remission of the disease persisted for 3 months and longer in only 7/20 of these cats. The inability to achieve long-term remission of the disease was ultimately associated with the occurrence of neurological disease in the absence of extensive abdominal lesions or with recurrence / persistence of extensive abdominal lesions in the presence of histological lesions in the brain and / or eyes. These findings suggest that FIPV has a greater tendency to spread from body cavities to the brain than previously thought, especially if given sufficient time. This spread most likely involves infected macrophages that enter the brain through small blood vessels in the meninges and ependyma.
In cats that developed neurological disease, this occurred either during treatment (CT05, CT08, CT22), or 2 (CT01, CT02, CT09), 3 (CT13) or 6 (CT10) weeks after treatment. The most likely explanation for this delay, as well as some therapeutic benefit of higher doses, was that part of GC376 was still able to penetrate the brain. GC376 levels in cerebrospinal fluid represented only 3% plasma in the brain 2 hours after subcutaneous injection at a dose of 10 mg / kg (unpublished data). Although the relative concentrations of drug in the brain of these cats were low, they were still 21.4 times higher than the levels required to inhibit virus replication in tissue culture. Based on this finding, it was hypothesized that higher doses would allow higher amounts of drug to enter the brain. This assumption was supported by the experience of two cats that showed neurological symptoms. Increasing the dose of GC376 to 50 mg / kg every 12 hours in one cat (CT01) resulted in a marked improvement but did not eliminate the symptoms of brain disease. Prolongation of treatment by almost 3 months at 15 mg / kg every 12 hours appeared to delay the progression of neurological symptoms in the second cat (CT12), while attempts to reduce the total daily dose in this cat to 10 mg / kg every 12 hours or 15 mg / kg caused worsening of neurological symptoms every 24 hours. This suggests that doses of 15 mg / kg every 12 hours or higher allowed sufficient GC376 to cross the blood-brain barrier to slow but not eliminate neurological symptoms.
The high incidence of central nervous system (CNS) disease in this study was higher than previously reported and was unexpected because cats with signs of brain or spinal cord involvement were excluded from the study. CNS diseases have been shown to be much more likely in older cats with dry or mixed FIP than in young cats with wet FIP. This suggests that FIPV can enter the brain of many cats if given sufficient time. Peritoneal-type macrophages appear to play a role in CNS infection because FIPV-infected cells in the brain of cats with neurological FIP are more similar to peritoneal macrophages than to resident brain macrophages. This should come as no surprise, as macrophages migrate to a variety of tissues, including the brain, to perform immune surveillance and are also targets for a variety of infectious agents, such as FIPV and HIV-1. Infected macrophages play a major role in the spread of the virus to the brain in HIV patients, and detection of the virus in the brain is possible within a few weeks of infection. However, neurological damage usually occurs at a later stage. Anti-HIV drugs also reduce the frequency of severe neurological disorders, similar to that observed in this study on FIPV and GC376. There are also alternative explanations. It is possible that extra-CNS involvement may inhibit the development of brain diseases and vice versa. It is common for CNS disease to occur in the absence of visceral disease and vice versa. Suppression of virus replication in non-neuronal tissues may also increase the positive selection of mutants that are more neurotropic or neurovirulent. However, evidence of the latter would require large studies using laboratory cats.
Certain forms of FIP appeared to affect treatment success. The behavior of GC376 in the treatment of ocular FIP was paradoxical because this form responded extremely well to GC376. Although the eye lesions responded to treatment, all three cats with the eye eventually succumbed to brain diseases, which supported a close anatomical relationship between the eye and the CNS. Chronic ileocecal and colon involvement and growth retardation in older cats in this study were also a poor prognosis. In many of these cats, abdominal effusion appeared only as a terminal manifestation of their disease. Host factors also associated with a reduced response to antiviral therapy for other viral infections, such as HCV, include age, gender, cirrhosis or liver fibrosis, race, or body weight.
The development of resistance is a major problem for any antiviral drug, but FIPV is rarely transmitted from cat to cat, and drug resistance, if it occurs, would be a problem only for individual treated cats and not for the entire population. Although viral resistance to GC376 has not been observed for up to 20 passages in vitro, suggesting that resistance cannot be easily acquired, long-term and repeated in vivo treatment may be a stronger selection factor. However, viral resistance did not appear to be responsible for relapses of abdominal disease in the five cats treated. These cats had granulomatous formations, often in the colon and ileo-cecal-colic lymph nodes, which could provide a protected site for viruses to persist. The protection of pathogens in granulomas is a well-documented phenomenon for mycobacteria and applies to other pathogens such as viruses. Liver disease (cirrhosis) from HCV infection also increases the risk of relapse and requires longer treatment, which also suggests that viruses may be protected from drugs when they are in certain protected areas. The formation of "protective granulomas" involves a large number of chemokines and cytokines and upregulation of chemokine receptors, addressins, selectins and integrins. The persistence of pathogens in these sheltered sites may require a higher dose of the drug and a longer duration of treatment.
Treatment failures may also be the result of the host's inability to elicit a protective immune response during periods of viral replication. Such failure has been observed in HCV infection in humans. T-cell mediated immunity plays an important role in the protective immunity that occurs in about 20% acute HCV infection and in the same or greater proportion of FIPV infection. The possible synergies between T-cell-mediated viral clearance and antiviral therapy in cats with FIP have yet to be investigated. A combination of antiviral drugs and T-cell immune stimulants in the treatment of FIP, such as the combination of interferons and ribavirin in the treatment of HCV infection, could also be beneficial.
Sustained remission in 6/7 cats treated for 12 weeks or longer was to some extent predictable. These cats were 3.3-4.4 months old at the time of acute abdominal symptoms (C15, C17, C18, CT21, CT23) or thoracic effusion FIP (CT20). This made them younger than all but three other cats in the study (CT8, CT16, CT21) and more resembled 16-week-old laboratory cats with acute onset effusion FIP, which responded well to GC376. The disease, if acute, gives the virus little time to penetrate the brain or eyes. The acute nature of their disease may also have allowed the infection to permanently disrupt any protective immune response. The seventh cat, CT04, was extreme compared to these six younger cats. At age 6.8, CT04 was the oldest cat in a study that experienced significant weight loss (30%) and mesenteric lymph node disease. CT04 suffered relapses of the disease requiring re-treatment, but all relapses were identical to baseline and did not involve the CNS. Cats with this form of FIP are known to undergo spontaneous remission, suggesting that there is a tipping point between immunity and disease. Cats CT04 and CT21 demonstrated the wise decision to restart treatment in relapse, provided that the relapses did not involve the eyes or nervous system and still respond to drugs.
The determination of the minimum duration of treatment was based on a gradual prolongation of the duration of treatment based on a favorable response to treatment. Based on experimental studies, 2 weeks of treatment were expected to be sufficient; it was therefore used as a starting point. However, this study indicated that the minimum treatment period was close to 12 weeks, which was surprisingly close to the usual 12-week period required to treat people with HCV with protease inhibitors. However, the duration of HCV treatment can range from 8 to 24 weeks in different people. The CT21 cat was healthy, active on the outside and grew after 12 weeks of treatment, but the total protein and white blood cell counts still did not return to normal, as in the other six cats. Nevertheless, it was decided to discontinue treatment after 17 weeks due to the long period of normal health. Whether longer-term treatment could prevent a relapse of the disease 13 weeks after the end of treatment, we can only imagine, but it raises doubts about how long the treatment period should be for some cats. The question also arises as to how long the remission period must be in order for us to declare that there has been a recovery, and not just a long-term remission. The longest disease-free period at the time of writing was more than 11 months, with five additional cats showing no signs of infection for 5-9 months. Based on clinical and histological evidence of neurological disease at the time of fatal relapses, it appears that the virus eventually reaches the brain and may be the most important limiting factor in FIP antiviral therapy.
Although only one-third of cats have survived for a long time, the 20 cats in this study provide the basis for future studies with GC376 and other antiviral drugs that will follow. Not all cats will be treatable, but that shouldn't stop the effort. In this limited study, almost all treated cats returned to normal health, albeit for only a few weeks or months. It is important to be aware of the universality of viral pathogens and to take advantage of the pioneering development of drugs that are used clinically in the treatment of human diseases such as HIV / AIDS, hepatitis C, MERS, SARS, Ebola and influenza.
Conclusions
Inhibition of FIPV 3CLpro by GC376 was shown to be effective under the study conditions and led to a reduction in virus replication and remission of disease symptoms in cats with naturally occurring FIP outside the CNS. However, persistent remission in this study occurred earlier in kittens less than 18 weeks of age with acute wet FIP or in cats with dry FIP limited to mesenteric lymph node and is less likely to occur in cats older than 18 weeks with dry, mixed or the ocular form of FIP. Failure to achieve permanent remission was associated with either a high incidence of neurological disease during or after treatment or a recurrence of abdominal lesions. Antiviral therapy appeared to slow the progression of neurological disease but failed to reverse it at the dose used in this study. The cause of recurrence of extra-neurological disease during treatment has not been determined, but was not related to mutations in the protease-binding region.
Footnotes
Received: 3.8.2017
Additional material: Informed consent form of the owner.
Conflict of interests: YK, KOC and WCG have patent claims on protease inhibitors. Other authors do not represent any potential conflicts of interest in connection with the research, authorship or publication of this article.
Financing: The main support for this study was made possible by a grant from the Morris Animal Foundation, Denver, CO, USA. Additional funding for technical support and animal care was provided by Philip Raskin Fund, Kansas City, SOCK FIP, National Institutes of Health grant R01AI109039, and the Center for Pet Health, University of California, Davis, CA, USA.
References
| 1. | Prokofiev, MM, Kochetkov, SN, Prassolov, VS. Therapy of HIV infection: current approaches and prospects. Acta Naturae 2016; 8: 23–32. Google Scholar | Crossref | Medline |
| 2. | Carter, W., Connelly, S., Struble, K. Reinventing HCV treatment: past and future perspectives. J Clin Pharmacol 2017; 57: 287–296. Google Scholar | Crossref | Medline |
| 3. | Kim, Y, Lovell, S, Tiew, KC. Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J Virol 2012; 86: 11754–11762. Google Scholar | Crossref | Medline |
| 4. | Kim, Y, Mandadapu, SR, Groutas, WC. Potent inhibition of feline coronaviruses with peptidyl compounds targeting coronavirus 3C-like protease. Antiviral Res 2013; 97: 161–168. Google Scholar | Crossref | Medline |
| 5. | Kim, Y, Shivanna, V, Narayanan, S. Broad-spectrum inhibitors against 3C-like proteases of feline coronaviruses and feline caliciviruses. J Virol 2015; 89: 4942–4950. Google Scholar | Crossref | Medline |
| 6. | Kim, Y, Liu, H, Galasiti Kankanamalage, AC. Reversal of the progression of fatal coronavirus infection in cats by a broad-spectrum coronavirus protease inhibitor. PLoS Pathog 2016; 12: e1005531. Google Scholar | Crossref | Medline | ISI |
| 7. | Miller, FG, Brody, H. What makes placebo-controlled trials unethical? Am J Bioethics 2002; 2: 3–9. Google Scholar | Crossref | Medline |
| 8. | Chiodo, GT, Tolle, SW, Bevan, L. Placebo-controlled trials good science or medical neglect? West J Med 2000; 172: 271–273. Google Scholar | Crossref | Medline |
| 9. | Van Kruiningen, HJ, Ryan, MJ, Shindel, NM. The classification of feline colitis. J Comp Pathol 1983: 93: 275–294. Google Scholar | Crossref | Medline | ISI |
| 10. | Pedersen, NC, Eckstrand, C, Liu, H. Levels of feline infectious peritonitis virus in blood, effusions, and various tissues and the role of lymphopenia in disease outcome following experimental infection. Vet Microbiol 2015; 175: 157–166. Google Scholar | Crossref | Medline | ISI |
| 11. | Pellerin, M, Lopez-Aquirre, Y, Penin, F. Hepatitis C virus quasispecies variability modulates nonstructural protein 5A transcriptional activation, pointing to cellular compartmentalization of virus-host interactions. J Virol 2004; 78: 4617–4627. Google Scholar | Crossref | Medline |
| 12. | Pedersen, NC, Allen, CE, Lyons, LA. Pathogenesis of feline enteric coronavirus infection. J Feline Med Surg 2008; 10: 529–541. Google Scholar | SAGE Journals | ISI |
| 13. | Pedersen, NC. A review of feline infectious peritonitis virus infection: 1963–2008. J Feline Med Surg 2009; 11: 225–258. Google Scholar |
| 14. | Pedersen, NC. An update on feline infectious peritonitis: virology and immunopathogenesis. Vet J 2014; 201: 123–132. Google Scholar | Crossref | Medline |
| 15. | Pedersen, NC, Liu, H, Durden, M. Natural resistance to experimental feline infectious peritonitis virus infection is decreased rather than increased by positive genetic selection. Vet Immunol Immunopathol 2016; 171: 17–20. Google Scholar | Crossref | Medline | ISI |
| 16. | Pedersen, NC, Liu, H, Gandolfi, B. The influence of age and genetics on natural resistance to experimentally induced feline infectious peritonitis. Vet Immunol Immunopathol 2014; 162: 33–40. Google Scholar | Crossref | Medline |
| 17. | Clifford, DB. HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med 2008; 16: 94–98. Google Scholar | Medline |
| 18. | Foley, JE, Lapointe, JM, Koblik, P. Diagnostic features of clinical neurologic feline infectious peritonitis. J Vet Intern Med 1998; 12: 415–423. Google Scholar | Crossref | Medline | ISI |
| 19. | Mesquita, LP, Hora, AS, de Siqueira, A. Glial response in the central nervous system of cats with feline infectious peritonitis. J Feline Med Surg 2016; 18: 1023–1030. Google Scholar | SAGE Journals | ISI |
| 20. | Pedersen, NC. Feline infectious peritonitis: something old, something new. Feline Pract 1976; 6: 42–51. Google Scholar |
| 21. | Valcour, V, Sithinamsuwan, P, Letendre, S. Pathogenesis of HIV in the central nervous system. Curr HIV / AIDS Rep 2011; 8: 54–61. Google Scholar | Crossref | Medline |
| 22. | Joseph, SB, Arrildt, KT, Sturdevant, CB. HIV-1 target cells in the CNS. J Neurovirol 2015; 21: 276–289. Google Scholar | Crossref | Medline |
| 23. | Spudich, S, Gonzalez-Scarano, F. HIV-1-related central nervous system disase: current issues in pathogenesis, diagnosis and treatment. Cold Spring Harb Perspect Med 2012; 2: a007120 Google Scholar | Crossref | Medline |
| 24. | Cavalcante, LN, Lyra, AC. Predictive factors associated with hepatitis C antiviral therapy response. World J Hepatology 2015; 7: 1617–1631. Google Scholar | Crossref | Medline |
| 25. | Saunders, BM, Cooper, AM. Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunol Cell Biol 2000; 78: 334–334. Google Scholar | Crossref | Medline |
| 26. | Smyk-Pearson, S, Tester, IA, Klarquist, J. Spontaneous recovery in acute human hepatitis C virus infection: functional T-cell thresholds and relative importance of CD4 help. J Virol 2008; 82: 1827–1837. Google Scholar | Crossref | Medline |
| 27. | Legendre, AM, Bartges, JW. Effect of polyprenyl immunostimulant on the survival times of three cats with the dry form of feline infectious peritonitis. J Feline Med Surg 2009; 11: 624–626. Google Scholar | SAGE Journals | ISI |
| 28. | Legendre, AM, Kuritz, T, Galyon, G. Polyprenyl immunostimulant treatment of cats with presumptive non-effusive feline infectious peritonitis in a field study. Front Vet Sci 2017; 4: 7. Google Scholar | Crossref | Medline |


